NOCION is a biopharmaceutical company that is addressing vexing medical problems head on. We are developing “nocions”—a new kind of therapy that selectively affects actively firing nociceptors—to provide targeted, robust and sustained relief for the treatment of serious conditions including cough, itch, pain and inflammation.

Create a nocion by ionizing and optimizing a proven sodium channel blocker

Nocions enter inflamed nociceptors through large-pore channels
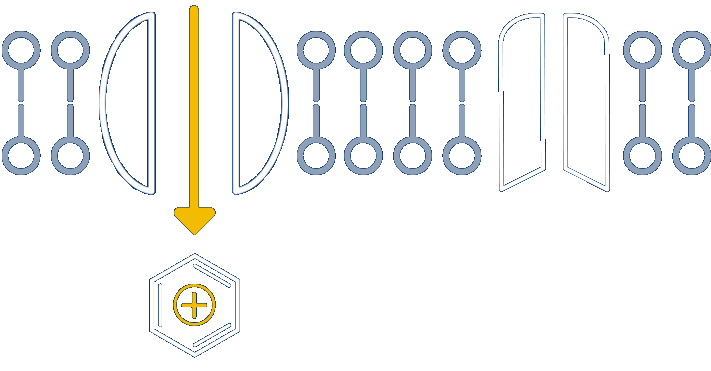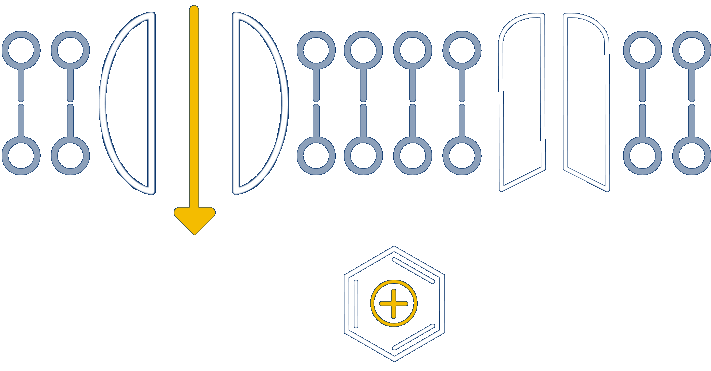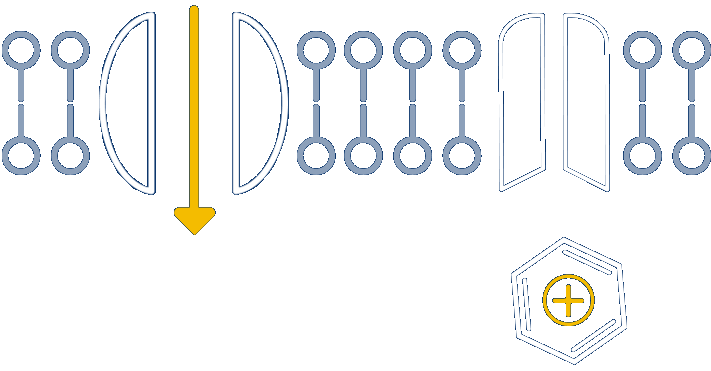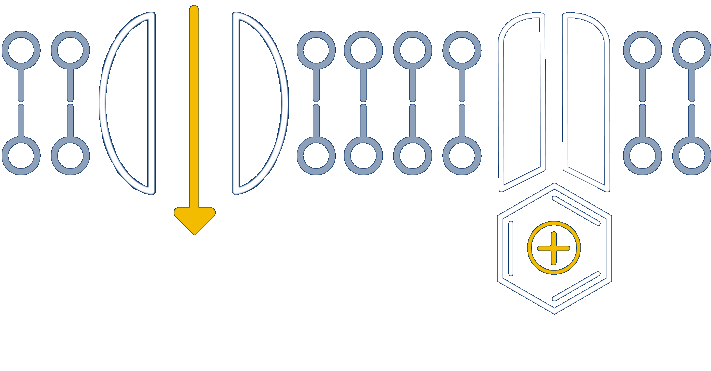
Nocions get trapped inside the inflamed nociceptors, inhibiting sodium channels and providing long-acting relief
Anchored in the well-understood physiology of nociceptors, our novel platform develops small molecules called nocions, which preferentially target activated sensory neurons that are responding to external insult. These inflamed sensory neurons are a source of cough, itch, pain and inflammation. More specifically, nocions are designed to only enter activated nociceptors via large-pore channels on these neurons that open in response to insult.
Once inside, nocions block all sodium channels. And because nocions are charged, they do not enter non-nociceptors or redistribute from nociceptors to local cells by passive diffusion. Thus, nocions are essentially trapped inside target cells to provide a targeted, robust and sustained effect that cannot be achieved with traditional small molecule anesthetics.
Other investigatory approaches to treating neurogenic inflammation include trying to develop molecules that either selectively block Nav 1.7 or antagonize large-pore channels. Both these approaches have been met with limited success due to undesired side effects and/or insufficient efficacy. In contrast, nocions only target activated sensory neurons using large-pore channels as doorways in and block all sodium channels in these cells.
Nocions offer a number of potential benefits vs standard sodium channel blockers, including:
Our focused platform may be applied to a broad range of applications. We’re actively finalizing leads to create a suite of product candidates intended to treat a wide variety of conditions.
Chronic cough is a significant unmet medical need with no proven standard of care. Nocions may provide benefit to patients with chronic idiopathic cough, post-infectious cough, IPF-cough, asthma-COPD overlap syndrome and additional inflammatory respiratory conditions.
Rick has been CEO at Nocion since August 2018. He has over two decades of experience in drug development across an array of platforms and disease states, and has been with several start-ups from founding through acquisition. Most recently, he was the Chief Scientific Officer and a founder of Civitas Therapeutics. Civitas was acquired by Acorda, whereupon he became their Chief Technology Officer and Civitas’ lead asset—a novel dry powder inhalation therapy to treat motor issues in Parkinson’s patients—has been approved by the FDA as InbrijaTM.
Rick was previously the Chief Scientific Officer and Sr. Vice President of Research and Development at Pulmatrix. He was previously the Vice President of Research and Development at Alkermes, overseeing many facets of product development across pulmonary, injectable and oral platforms. Rick was an original member of Advanced Inhalation Research (AIR®), where he oversaw product development.
Rick held several academic posts prior to joining AIR. He received his B.Sc. in Chemical Engineering from the University of Calgary and his S.M. and Ph.D. in Chemical Engineering from the Massachusetts Institute of Technology (MIT).
Peter joined Nocion as Chief Financial Officer in March 2021, with 3 decades of experience in the industry, in areas of expertise including Fundraising (public and private), Accounting, Financial Planning and Analysis, Facilities (construction and management), Human Resources, Executive Compensation, Investor Relations, and helping incubate and manage companies from an early stage through to various exits.
After training as a CPA, Peter was Controller at Genzyme Tissue Repair and Director of Finance at Millennium Pharmaceuticals, and then either Acting or Full-Time CFO at several companies including Civitas Therapeutics, Acceleron Pharma, Visterra, Aveo, Kala, and others.
Peter received his B.S. in Accounting from Bentley University and his M.B.A. from Babson College.
Jim has been CSO at Nocion since July 2018. He has over 20 years leadership experience in drug discovery in multiple therapeutic areas, working in organizations from biotech startups to large pharma. Most recently, Jim lead GSK’s Innovation Hub in Cambridge after serving as Vice President and Head of GSK’s Sirtuin Discovery Performance Unit.
Prior to GSK, Jim was Vice President of Research for Surface Logix and the Executive Project Director and Chief Biology Advisor for NitroMed. Previously, Jim was the Group Director, Allergy and Respiratory Disease and Senior Director of Pharmacology at UCB Pharma where he was responsible for UCB’s global allergy and respiratory franchise research pipeline.
Prior to joining industry, Jim was an Assistant Professor of Medicine at Johns Hopkins University, where he also did his post-doctoral research. Jim received his B.Sc. in Pharmacology from the University of Aberdeen in Scotland and his Ph.D. in Neurobiology from University College London.
Matt joined Nocion as Chief Medical Officer in April 2025. Before joining Nocion, Matt served as Chief Medical Officer at Chemomab, Inc., where he spearheaded the development of a novel monoclonal antibody for fibrotic and inflammatory diseases, culminating in a successful end-of-phase 2 meeting with the FDA. Previously he was Vice President, Clinical Development & Medical Affairs, Specialty Pharma at Boehringer Ingelheim where he was responsible for developing new drugs for oncology, immunology, pulmonary, and CNS diseases. In a prior role at Novartis, Matt was Vice President & Head, Immunology and Dermatology Medical Unit, where he oversaw medical affairs and clinical development for Cosentyx®, Ilaris®, and Zortress®.
Earlier in his career, Matt led the medical affairs unit at Sandoz supporting the biosimilar, biopharmaceutical and generics businesses. Matt also previously held clinical development leadership roles across geographies and therapeutic areas at Reata, Fibrogen, Abbott Labs, and Schering Plough.
Matt received a B.A. from Vassar College, an M.D. from the University of California School of Medicine, Los Angeles and an MBA from the J. L. Kellogg Graduate School of Management at Northwestern University. He is board certified in internal medicine.
Joan joined Nocion as the Senior Vice President of Clinical Operations in April 2024. Prior to joining Nocion, Joan served as the SVP of Clinical Operation at Bellus Health (now GSK) and established their global late-stage drug development strategy for refractory chronic cough, completed their successful Phase 2b study and initiated a program of Phase 3 studies. She has a unique combination of experience with large pharmaceutical companies (Astra Zeneca, DuPont Pharma, Takeda), biotech companies (Bellus Health, Agenus) and contract research organizations (Parexel, UBC, Quotient Sciences) in multiple therapeutic areas spanning over 30 years.
Joan is a licensed Medical Technologist (B.S., University of Delaware) and an MS in Clinical Chemistry (West Chester University) and holds a Six Sigma Black Belt (Villanova).
Chairman of the Board
President and CEO, Nocion Therapeutics
Principal, Monograph Capital
General Partner, Canaan Partners
Investment Professional, Morningside Technology Advisory
Managing Partner, Arkin Bio Capital
Consultant Respiratory Physician and Professor of Respiratory Medicine at King’s College Hospital and King’s College London
Independent Life Sciences Industry Consultant
Professor of Medicine,
Johns Hopkins University
Professor of Respiratory Medicine, Faculty of Medicine, National Heart and Lung Institute,
Professor of Respiratory Medicine, Centre for Experimental Medicine, School of Medicine, Dentistry and Biomedical Sciences, Queen’s University Belfast
Head of Respiratory Medicine and the Centre for Cardiovascular and
University of Hull
Professor of Medicine and Pediatrics in the Division of Allergy and Immunology, University of South Florida at Johns Hopkins All Children’s Hospital
Professor of Respiratory Medicine at the University of Manchester and Honorary Consultant at Manchester University NHS Foundation Trust
Professor of Medicine,
Johns Hopkins University